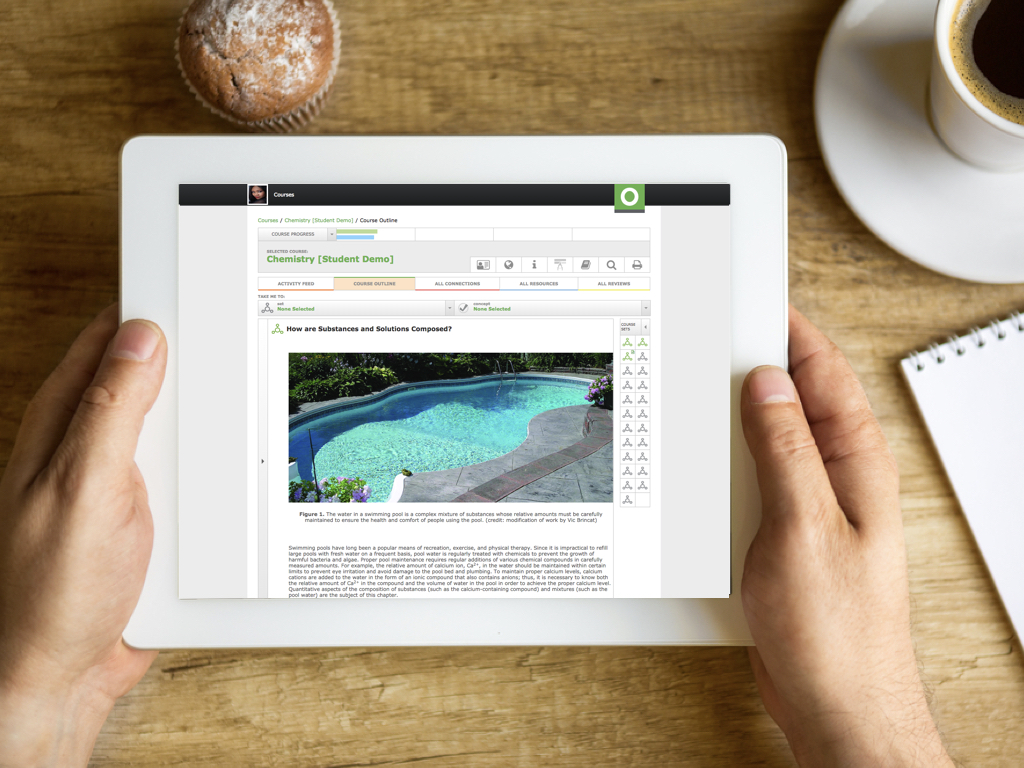
Introduction to Chemistry
The comprehensive contents from this book, combined with Odigia’s Teaching and Learning Tools have everything you need to engage, collaborate, track and assess your students.
This course includes:
165
practice questions
111
engagement activities
Helping Teachers Do What They Do Best: Teach

Customize
Use our courses as is or easily customize them to fit your teaching style and the needs of your students. You can add your favorite resources, hide and show our existing content and pre-built assessments, or make them your own. Everything your students need, in one place!

Engage and Collaborate
Odigia combines learning materials, discussions, and tools to create a familiar social experience for students allowing you to easily connect and redirect students attention.

Track
See how much time students are spending on different areas of the course, which areas are creating the most amount of engagement and identify topics the students are struggling with. Flag and provide feedback on assignments to proactively meet individual students' needs.

Assess
Game theory allows students to monitor their progress visually and motivates them to stay on track. Students can see exactly what activities they need to complete, which ones have been flagged and compare their progress against the overall class.
Introduction to Chemistry Course Outline
Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered: Concepts Covered:What is the Scientific Method?
How are things measured scientifically?
What is energy? How is it measured?
What is matter?
What are atoms? How are they structured?
How are electrons arranged in atoms?
What is nuclear chemistry?
What are ions? How can ions be combined into ionic compounds?
What are covalent compounds? How is the shape and attractions determined for covalent compounds?
What are chemical reactions? How are chemical reactions represented and classified?
Who was Avogadro? What are units for quantifying atoms and compounds?
What is Stoichiometry? How are quantities calculated in chemical reactions?
What is a gas? How do properties of gases relate to one another?
What is a solution? What are its properties?
What is chemical equilibrium? How is it achieved in chemical reactions?
What are acids and bases? How do they react with other substances?
About the book
Introduction to Chemistry

This is the Introduction to Chemistry course developed by the Community College Consortium of Bioscience Credentials. Created by subject matter experts under the Department of Labor’s Trade Adjustment Assistance Community College Career Training (TAACCCT) grant program, these courses can be used as-is or modified to meet your own learning objectives and teaching style.
